Production of recombinant proteins in non-native expression hosts (such as plants) necessitates extensive characterization work to confirm the appropriate functionality of the recombinant molecule. Our group has specialized in bioanalytic techniques that enable us to assess the integrity and efficacy of these proteins.
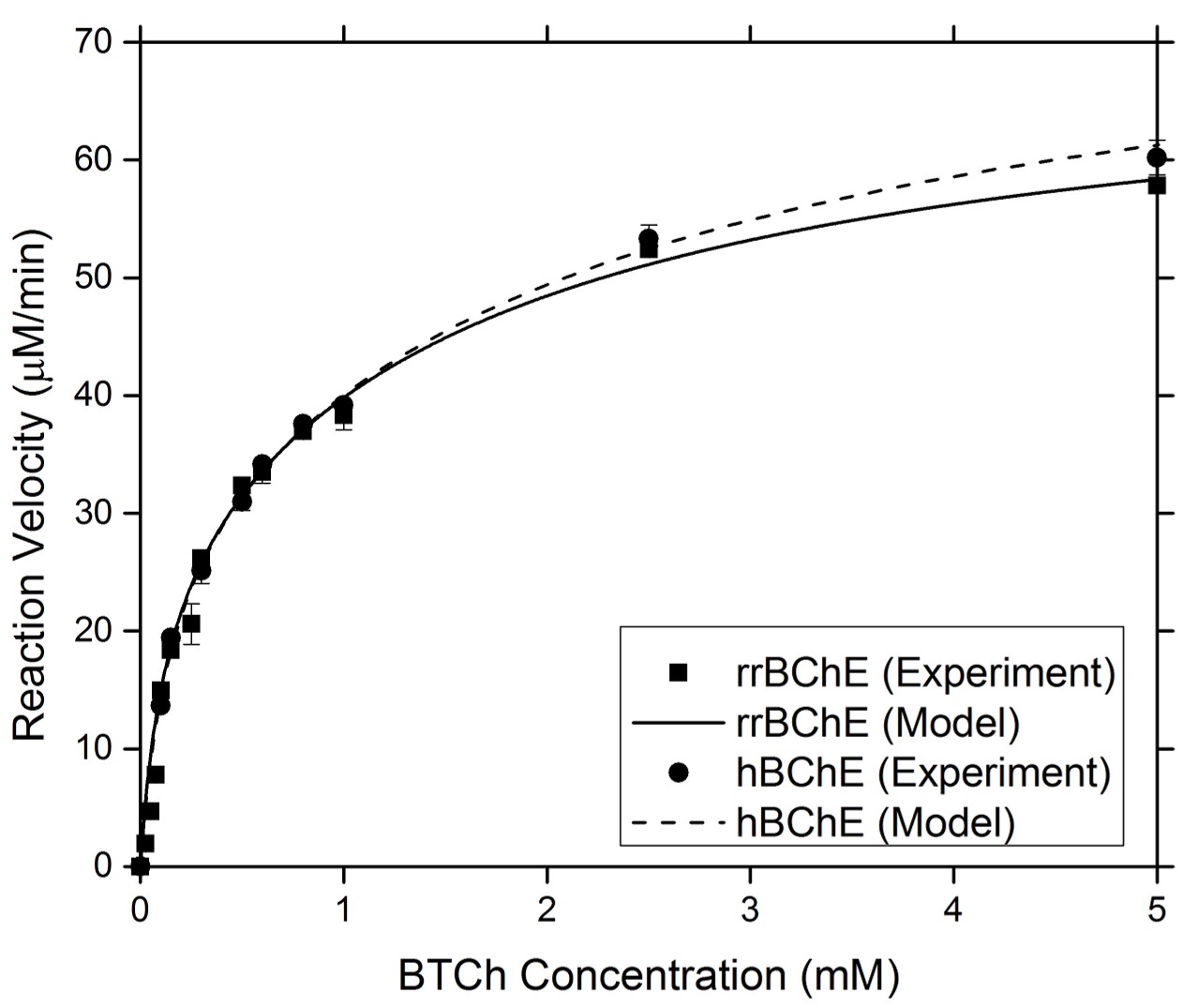
Enzyme Kinetics
Our group has worked extensively to produce recombinant enzymes in both plant hosts. To assess the comparability between the recombinant and native forms of an enzyme, we have studied the kinetic behavior of the enzyme in response to different environments. We also used a modified form of Michaelis Menten kinetics to determine kinetic parameters that confirm the comparable kinetic behavior of the recombinant and native molecules.
Binding Kinetics
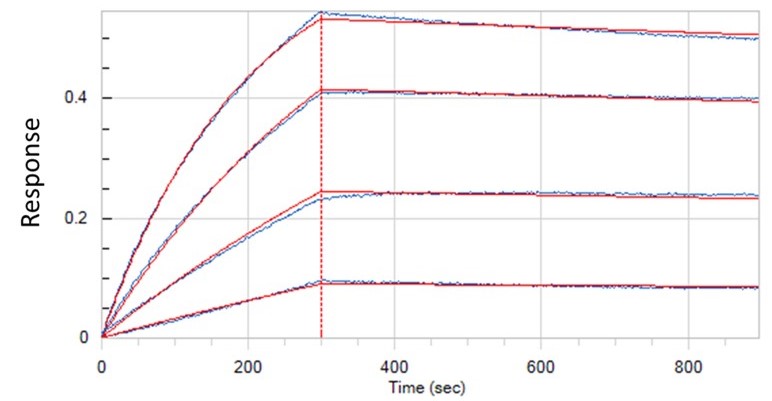
- Real-time binding kinetics information, allowing calculation of association and dissociation rates as well as binding affinity.
- Save precious samples, allowing binding kinetics study with minimal amount of sample within nano-molar range.
- High throughput (BLI), up to 96 sensor tips can be simultaneously dipped into solution of binding partners, allowing binding kinetics measurements and binding partner screening within brief time period.
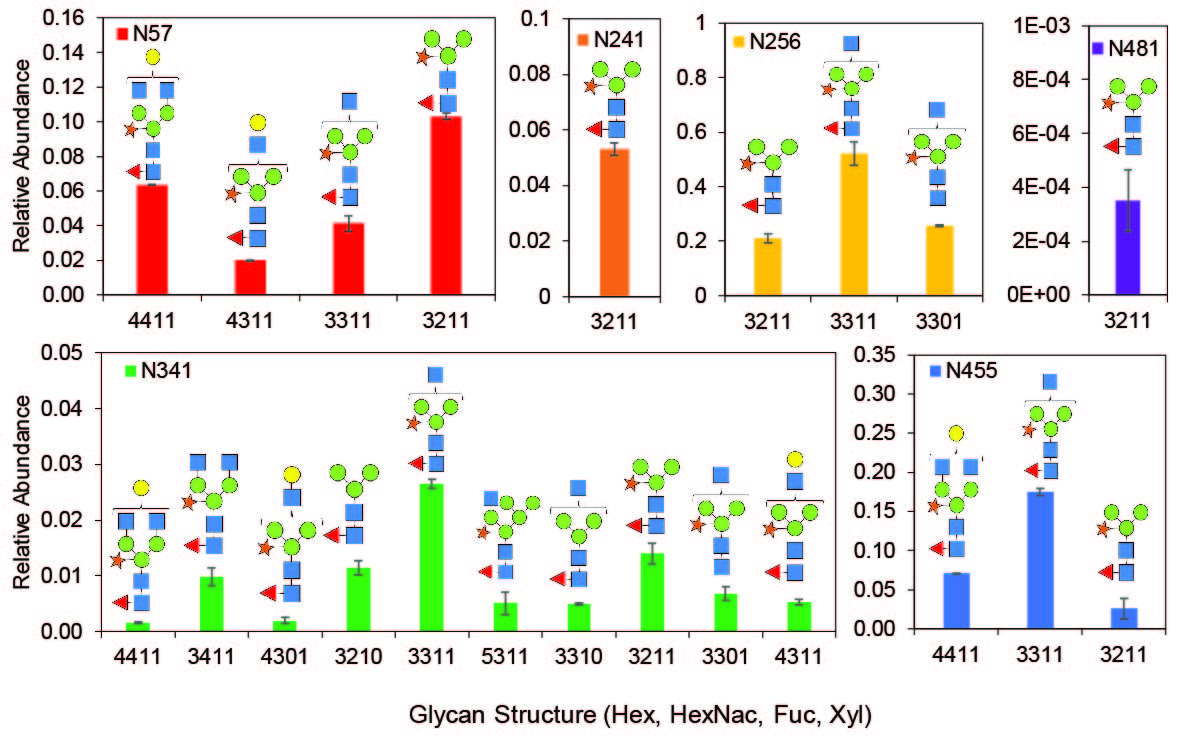
Glycan Characterization
Plant-made glycoproteins generally contain plant-specific glycan structures, which may differ from proteins produced in other expression hosts. While there has been no definitive evidence of immunogenicity of plant-made glycans (Shaaltiel and Tekoah, Nature Biotechnology, 2016), it is important to understand these structures as they may impact the activity, biodistribution, or pharmacokinetic stability of the molecule. With help from our collaborators, we have characterized the structure and distribution of N-glycans present on our plant-made recombinant proteins.
These N-glycans can be controlled using a combination of genetic engineering of the expression host and bioprocessing methods, all of which our group uses to our advantage. For example, in addition to wild-type glycoform plants, our group also works with plants genetically engineered for glycoform modification.
Electron Microscopy
Our group uses confocal and electron microscopy techniques for visualization and structural analysis of our biopharmaceutical intermediates and products.
